One of the biggest societal challenges we face today, pointed out in reports of many governmental and pan-governmental organisations, is the pandemic proportions antibiotic resistant bacteria start to show. These reports also mention a gap between the rate of resistance occurrence and the development of novel antibiotics, explained by the lack of investments from pharmaceutical companies. The development of novel antibiotics forms a major challenge, both in Europe and worldwide, implying that they will have to be accessible to patients in the third and fourth world. Thus, their production process will have to be cost competitive compared to established drugs, making new drug therapies more economically attractive.
Forecast Deaths Attributable to Antibiotic Resistance in 2050
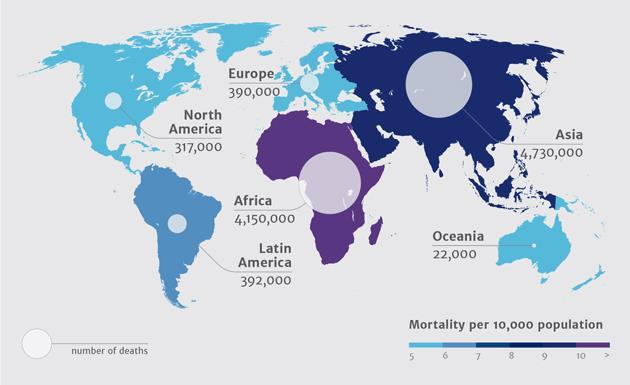
Source: Review on Antimicrobial Resistance – Tackling a Crisis for the Health and Wealth of Nations (2014)
A second important burden on our healthcare system are orphan diseases. Orphan diseases are rare diseases that do not affect many people, and hence are considered not profitable enough by the pharmaceutical sector. Therefore little effort is put into the discovery and development of novel medicines that alleviate or cure them. Many orphan diseases are genetic disorders, such as cystic fibrosis or ALS (amyotrophic lateral sclerosis), caused by DNA mutations that result in a stop codon and premature translation arrest. RNA interacting molecules can alleviate such premature arrests, reducing the effect of the mutation and forming a cure.
"A disease may be rare, but hope should not be..."
Although both medical problems seemingly are independent, on an RNA level they can both be affected by the same molecules, i.e. by aminoglycosides. Aminoglycosides (AGs) are highly potent, broad-spectrum antibiotics. Also, they can be used as post-transcriptional control (PTC) agents for treating genetic disorders and they showed to be effective as anti-virals, e.g. in HIV treatment. While AGs are very promising, some issues need to be addressed to exploit and secure their full potential in healthcare industry, i.e. structural diversity and improved production processes are needed.
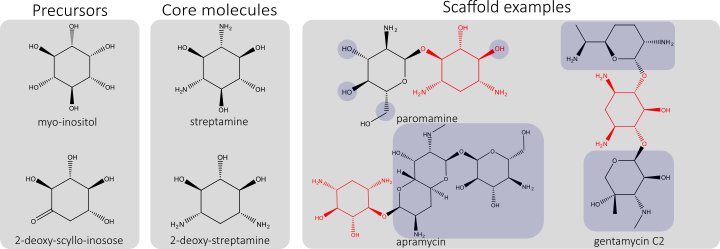
At InBio.be we are currently developing industrially competitive ways for AG production, by selecting and fine-tuning pathways in metabolically engineered, robust chassis strains. As such we strive to develop a platform technology for the versatile production of a plethora of these promising molecules. Basic AG structures, starting from precursors, are converted in core molecules and further modified with functional groups to form AG scaffolds. These scaffolds can be further decorated, resulting in a specific AG (figure below, cores in red, examples of structural modifications shaded).
For more information about AGs and possible collaborations, please contact Prof. Wim Soetaert or Dr. Sofie De Maeseneire.
